28 electrons
Nickel atoms have 28 electrons and the shell structure is 2.8.
Curtis Strite
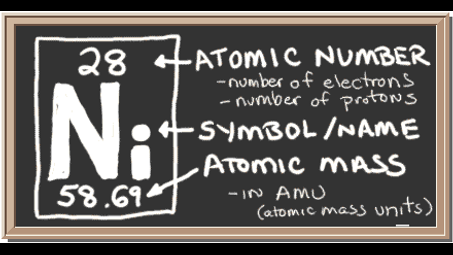
The element Nickel (Ni) has 10 core electrons, which correspond to the electrons in the inner electron shells that are not involved in chemical bonding. Core electrons shield the valence electrons from the positive charge of the nucleus.
Wiki User
∙ 9y agoThis is a chemical element. You can find the how many electron in a single atom by using a Periodic Table.
Armia Motejadded
28
Manreet Dhaliwal
18
Wiki User
∙ 11y agoit have 2
Add your answer:
How many core electrons does argon have?
Argon has 18 core electrons. This is because the atomic number of argon is 18, and the number of core electrons is equal to the number of electrons in the nearest noble gas configuration, which in this case is neon (10 core electrons), plus the number of electrons in the next energy level, which is 8 for argon.
How many electrons does Ni 3 plus have?
Ni3+ has 23 electrons. This is because Nickel (Ni) normally has 28 electrons, but when it loses 3 electrons to become Ni3+, its electron count decreases to 23.
How does the energy of core electrons compare with that of valence electrons?
The energy of the valence electrons is greater then the energy of the core electrons.APEX
What phrase describes core electrons?
electrons that are closer to the nucleus!..apex//
Where are the core electrons located?
Core electrons are located in the inner electron shells of an atom, closest to the nucleus. These electrons are tightly bound to the nucleus and are not typically involved in chemical reactions or bonding with other atoms.
How many core electrons are in Cd?
Cadmium (Cd) has 48 electrons, and its electron configuration is [Kr] 4d10 5s2. This means that the core electrons in cadmium are those in the inner shells, which are the 36 electrons from the noble gas krypton (Kr). Therefore, cadmium has 36 core electrons.
How many core electrons are in Ion?
Oxygen has 6 core electrons.
How many core electrons does germanium?
Germanium has 28 core electrons.
How many core electrons in sn?
Tin has 46 core electrons.
How many core oxygen electrons?
Oxygen as 2 core electrons and 6 valence electrons.
How many core electrons does nitrogen have?
Nitrogen atoms have two core electrons.
How many core electrons are in oxygen ion?
Oxygen has 6 core electrons.
How many core electrons in a silicon?
Silicon has a total of 10 core electrons and 4 valence electrons.
How many core electrons are in bromine?
2,8,18,7... 28 core electrons 7 valence electrons.
How many electrons are core electrons in the element cesium?
In cesium, there are 54 core electrons. This is calculated by subtracting the number of valence electrons (1) from the total number of electrons in cesium, which is 55. Core electrons are the inner electrons that are not involved in chemical bonding.
How many core electrons does phosphorus have?
well core electrons is the number of total electrons minus valence electrons so.......Phosphorus has 18 electrons and 5 valence electrons so 18 - 5 = 13 so there are 13 core electrons
How many core electron does n3- have?
2 core electrons and 8 valence electrons are there in N3- ion.
