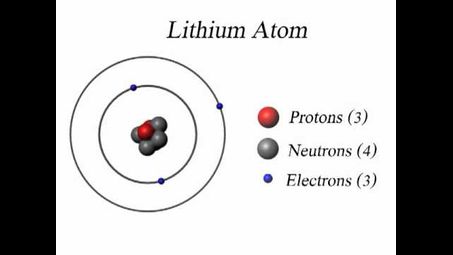
Lithium by default contains 3 protons and 4 neutrons so its Atomic Mass is 6.941 amu (atomic mass units)
What else can I help you with?
How many protons are in a lithium nucleus?
The atomic number of lithium is 3. Therefor there are 3 protons and neutron in the nucleus of lithium. Lithium also has 4 neutrons. atomic # = protons and neutrons neutrons = rounded atomic mass - atomic number Sources- 7th grade Acc. Science
The nucleus of an atom contains the?
The nucleus of an atom contains protons and neutrons. Protons have a positive charge, while neutrons have no charge. The nucleus is the central core of an atom where most of its mass is concentrated.
Does the nucleus contain neutrons and electrons?
No, the nucleus contains protons and neutrons. Electrons are outside of the nucleus.
The central part of an atom that contains protons and neutrons?
nucleus
Name a part of the atom that contains protons and neutrons?
The atomic nucleus is the part of the atom that contains protons and neutrons.
How many protons are in a lithium nucleus?
The atomic number of lithium is 3. Therefor there are 3 protons and neutron in the nucleus of lithium. Lithium also has 4 neutrons. atomic # = protons and neutrons neutrons = rounded atomic mass - atomic number Sources- 7th grade Acc. Science
What does a lithium atom contain?
A lithium atom contains three protons, three electrons, and usually four neutrons in its nucleus. The electrons occupy energy levels around the nucleus in a specific configuration.
The nucleus of an atom contains the?
The nucleus of an atom contains protons and neutrons. Protons have a positive charge, while neutrons have no charge. The nucleus is the central core of an atom where most of its mass is concentrated.
The nucleus of an atom contains what two particles?
nucleus....contains protons & neutrons
How many protrons and neutrons does lithium have?
Lithium has 3 protons and typically 4 neutrons in its nucleus.
Does the nucleus contain neutrons and electrons?
No, the nucleus contains protons and neutrons. Electrons are outside of the nucleus.
The central part of an atom that contains protons and neutrons?
nucleus
What parts of an atom are found in the nucleus?
The nucleus of an atom contains neutrons and protons and protons, in turn, contain quarks.
The small central part of an atom?
The "dense" central portion of an atom is called the nucleus. The nucleus of an atom contains neutrons and protons.
What contains the protons and neutrons in an atom?
The nucleus
What contains an atom's protons and neutrons?
the nucleus
Name a part of the atom that contains protons and neutrons?
The atomic nucleus is the part of the atom that contains protons and neutrons.
