A carboxylic acid has the form:
R-COOH or R-C(=O)OH
Where R is a hydrocarbon chain.
A diagram from Wikipedia: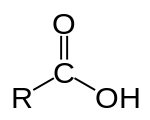
The Hydrogen attached to the OH the one released by the acid.
In reality, both bonds to the oxygen probably have some aspect of being 1 1/2 bonds rather than true double bonds (resonance), although, apparently in the acid form they are of different lengths. However, the bond lengths equalize when in the conjugate base form.
What else can I help you with?
What is the name of the ion formed form a carboxylic acid?
The generic term is carboxylate; more specifically, it's the root name of the compound with "-ic acid" replaced by "-ate": acetic acid -> acetate; benzoic acid -> benzoate, etc.
Does r-cooh behave as an acid?
Yes, R-COOH (carboxylic acid) behaves as an acid because the hydrogen atom attached to the oxygen is acidic and can dissociate as a proton (H+) in solution. This dissociation leads to the formation of the carboxylate ion (R-COO-).
Why the valency is two for oxalic acid?
Oxalic acid has a valency of two because it can donate or accept two hydrogen ions in a chemical reaction. Each carboxylic group in oxalic acid can release one hydrogen ion, giving it a valency of two.
What is the difference between a carboxylate and a carboxylic acid in terms of their chemical properties and reactivity towards other compounds?
A carboxylate is the ionized form of a carboxylic acid, meaning it has lost a hydrogen ion. Carboxylates are generally more stable and less reactive than carboxylic acids. Carboxylic acids are more acidic and tend to react with other compounds to form salts or esters.
When an acid is added to water what ion is released?
When an acid is added to water, hydrogen ions (H+) are released. This is what gives acidic solutions their characteristic properties.
What is the name of the ion formed form a carboxylic acid?
The generic term is carboxylate; more specifically, it's the root name of the compound with "-ic acid" replaced by "-ate": acetic acid -> acetate; benzoic acid -> benzoate, etc.
Does r-cooh behave as an acid?
Yes, R-COOH (carboxylic acid) behaves as an acid because the hydrogen atom attached to the oxygen is acidic and can dissociate as a proton (H+) in solution. This dissociation leads to the formation of the carboxylate ion (R-COO-).
Why the valency is two for oxalic acid?
Oxalic acid has a valency of two because it can donate or accept two hydrogen ions in a chemical reaction. Each carboxylic group in oxalic acid can release one hydrogen ion, giving it a valency of two.
What is the difference between a carboxylate and a carboxylic acid in terms of their chemical properties and reactivity towards other compounds?
A carboxylate is the ionized form of a carboxylic acid, meaning it has lost a hydrogen ion. Carboxylates are generally more stable and less reactive than carboxylic acids. Carboxylic acids are more acidic and tend to react with other compounds to form salts or esters.
Is Acid organic or inorganic compound?
there are organic acids and inorganic acids
Can chromic acid have one hydrogen acid ion?
This is possible when the ion is Hydrogen chromate or HCrO4-
When an acid is added to water what ion is released?
When an acid is added to water, hydrogen ions (H+) are released. This is what gives acidic solutions their characteristic properties.
Why is the hydrogen atom of the -OH group on a carboxylic acid the most acidic hydrogen in this type of molecule?
In a carboxylic acid, all hydrogen atoms are attached to carbon atoms except from the one attached to the oxygen atom, on the end of the molecule. This hydrogen ionises on contact with water, producing an H+ ion and an O- ion, which remains attached to the rest of the molecule.Only this hydrogen atom will ever ionise, if hydrogen is attached to carbon it will never ionise, no matter what.
What is the process for formingester bonds in organic chemistry?
In organic chemistry, ester bonds are formed through a reaction called esterification. This process involves the reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst. The carboxylic acid donates a hydrogen ion (H) to the alcohol, forming water as a byproduct. The remaining components then combine to form an ester bond, linking the carboxylic acid and alcohol molecules together.
What is salt of naphthalic acid?
The salt of naphthalene carboxylic acid, also known as naphthalic acid, is formed when a naphthalic acid molecule donates a hydrogen ion to a base molecule. This results in the formation of a salt compound with a positively charged ion and a negatively charged ion.
What is the term for an acid that can donate only one hydrogen ion?
The term for an acid that can donate only one hydrogen ion is monoprotic acid.
Is HC2H3O2 an acid base or salt?
If the chemical formula refers to 'Ethanoic (Acetic) Acid , then as the name suggests it is an ACID. HC2H3O2 as ethanoic acid is usually written as ' CH3-C(=O)OH'. or 'CH3COOH' Being a Carboxylic (Organic/Fatty) Acid , the acid functional group is always written as 'R-COOH'. By this method it indicates to chemists world wide that it is a carboxylic acid. The 'R' is the rest of the organic molecule.
