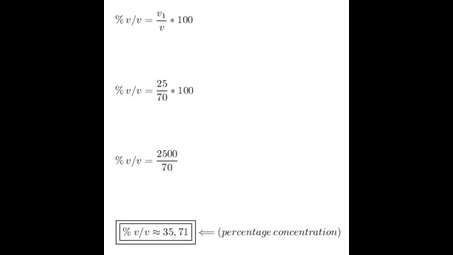
What is the percent by volume of a solution formed by mixing 25mL of isopropanol with 45 mL of water?
% V / V (percentage of volume per volume) =?
V1 (solute volume) = 25 mL
V (volume of the solution) = solute + solvent = 25 mL + 45 mL = 70 mL
We apply the data to the formula of volume percentage of the solute per volume of solution, we will have:
What else can I help you with?
Is mixing sugar with iodine solution a chemical change or a physical change?
Mixing sugar with iodine solution is a physical change because no new substances are formed. The sugar and iodine solution retain their individual chemical properties even when mixed together.
What is the volume percent concentration of hexane in a solution made by mixing 50.0 mL of hexane with 1.0 L of pentane?
To calculate the volume percent concentration of hexane in the solution, you need to determine the total volume of the solution first. Mixing 50.0 mL of hexane with 1.0 L of pentane gives a total volume of 1.05 L (1000 mL + 50 mL). The volume percent concentration of hexane can be calculated as (volume of hexane / total volume) x 100. This gives (50 mL / 1050 mL) x 100 = 4.76% volume percent of hexane in the solution.
A chemist is making 200 L of a solution that is 62 percent acid He is mixing an 80 percent acid solution with a 30 percent acid solution How much of the 80 percent acid solution will he use?
The chemist will use 100 liters of the 80% acid solution and 100 liters of the 30% acid solution to make a 200-liter solution that is 62% acid. The amount of acid in the 80% solution will be 0.8 * 100 = 80 liters, and in the 30% solution, it will be 0.3 * 100 = 30 liters.
What is the volume percent concentration of hexadecimal in a solution made by mixing 50.0 ml of hexadecimal with 1.0 like of pentane?
To calculate the volume percent concentration of a component in a solution, you need to know the total volume of the solution. In this case, the total volume after mixing would be 50.0 ml + 1.0 ml = 51.0 ml. To find the volume percent of hexadecimal, you would take the volume of hexadecimal (50.0 ml) divided by the total volume of the solution (51.0 ml) and multiply by 100. This gives you a volume percent concentration of hexadecimal in the solution.
What solutions make a purple precipitate?
A purple precipitate can be formed by mixing potassium permanganate solution with iron(II) sulfate solution. This reaction produces a solid manganese dioxide precipitate.
How many liters of a 90 percent acid solution must be mixed with a 15 percent acid solution to get 600 L of a 80 percent solution?
Mixing 80 liters of 15% solution and 520 liters of 90% solution will give 600 liters of 80% solution.
Is it possible to mix a 10 percent acid solution and a 40 percent acid solution to obtain a 60 percent acid solution?
No. The reulting concentration (percent) must be between the two components. So, with the two acids you are mixing, you cannot get an acid that is less than 10% or more than 40%
Is mixing sugar with iodine solution a chemical change or a physical change?
Mixing sugar with iodine solution is a physical change because no new substances are formed. The sugar and iodine solution retain their individual chemical properties even when mixed together.
What is solution in solid?
A solid solution is formed when two crystalline solids combine to form a crystal lattice. One example of a solid solution is copper and zinc mixing to create brass.
What is the volume percent concentration of hexane in a solution made by mixing 50.0 mL of hexane with 1.0 L of pentane?
To calculate the volume percent concentration of hexane in the solution, you need to determine the total volume of the solution first. Mixing 50.0 mL of hexane with 1.0 L of pentane gives a total volume of 1.05 L (1000 mL + 50 mL). The volume percent concentration of hexane can be calculated as (volume of hexane / total volume) x 100. This gives (50 mL / 1050 mL) x 100 = 4.76% volume percent of hexane in the solution.
What is solid solution?
A solid solution is formed when two crystalline solids combine to form a crystal lattice. One example of a solid solution is copper and zinc mixing to create brass.
A chemist is making 200 L of a solution that is 62 percent acid He is mixing an 80 percent acid solution with a 30 percent acid solution How much of the 80 percent acid solution will he use?
The chemist will use 100 liters of the 80% acid solution and 100 liters of the 30% acid solution to make a 200-liter solution that is 62% acid. The amount of acid in the 80% solution will be 0.8 * 100 = 80 liters, and in the 30% solution, it will be 0.3 * 100 = 30 liters.
What is the volume percent concentration of hexadecimal in a solution made by mixing 50.0 ml of hexadecimal with 1.0 like of pentane?
To calculate the volume percent concentration of a component in a solution, you need to know the total volume of the solution. In this case, the total volume after mixing would be 50.0 ml + 1.0 ml = 51.0 ml. To find the volume percent of hexadecimal, you would take the volume of hexadecimal (50.0 ml) divided by the total volume of the solution (51.0 ml) and multiply by 100. This gives you a volume percent concentration of hexadecimal in the solution.
How many ounces of the 50 percent alcohol solution should be mixed with the 1 percent alcohol solution to make 8 oz of the 20 percent alcohol solution?
Let x = ounces of 50% solution, and y = ounces of 1% solution. So that we have: 0.5x + 0.01y = 8(0.2) which is a linear equation in two variables, meaning there are infinitely many choices of mixing those solutions.
What solutions make a purple precipitate?
A purple precipitate can be formed by mixing potassium permanganate solution with iron(II) sulfate solution. This reaction produces a solid manganese dioxide precipitate.
Is mixing liquid soap and water a solution?
Yes, it is a solution.
How many gallons of a 90 percent antifreeze solution must be mixed with 90 gallons of 15 percent antifreeze to get a mixture that is 80 percent antifreeze?
Let M equal the gallons of the 90% mixing solution. Let F equal the gallons of the final solution. So:90 + M = F.Also, the number of gallons of pure antifreeze in the final will equal the sum of the gallons of antifreeze in the two mixing parts:Original solution: ( 90 gal )*(0.15) = 13.5 gal [0.15 represents 15%]Mixing solution: M*0.90Final solution: F*0.80So 13.5 + M*0.90 = F*0.80Now you have 2 linear equations and 2 unknowns, you can solve for M & F, using your favorite method: M = 585 and F = 675. Add 585 gallons of the 90% to get 675 gallons of 80% solution.
