A carboxylic acid has the form:
R-COOH or R-C(=O)OH
Where R is a hydrocarbon chain.
A diagram from Wikipedia: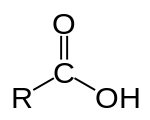
The Hydrogen attached to the OH the one released by the acid.
In reality, both bonds to the oxygen probably have some aspect of being 1 1/2 bonds rather than true double bonds (resonance), although, apparently in the acid form they are of different lengths. However, the bond lengths equalize when in the conjugate base form.
Wiki User
∙ 14y agoAdd your answer:
When an acid is added to water what ion is released?
Hydrogen ions.
What is the structural formula of formic acid in water?
When any carboxylic acid is place in water, hydrogen ion transfer occurs to produce hydronium ion and carboxylate ion. ( R-COOH + H20 = H30 + R-COO- ) So formic acid with the addition of water is HCOOH + H20 = H30 + HCOO-
Why is the hydrogen atom of the -OH group on a carboxylic acid the most acidic hydrogen in this type of molecule?
In a carboxylic acid, all hydrogen atoms are attached to carbon atoms except from the one attached to the oxygen atom, on the end of the molecule. This hydrogen ionises on contact with water, producing an H+ ion and an O- ion, which remains attached to the rest of the molecule.Only this hydrogen atom will ever ionise, if hydrogen is attached to carbon it will never ionise, no matter what.
What causes HCl to become an acid?
HCI is the chemical formula for Hydrochloric acid. HCI is composed of a hydrogen ion and a chloride ion. The hydrogen ion causes HCI to become an acid.
IS Hydrogen thiocyanate AN ACID?
Well since it contains hydrogen ion, it must be an acid.
When an acid is added to water what ion is released?
Hydrogen ions.
Can chromic acid have one hydrogen acid ion?
This is possible when the ion is Hydrogen chromate or HCrO4-
What is the structural formula of formic acid in water?
When any carboxylic acid is place in water, hydrogen ion transfer occurs to produce hydronium ion and carboxylate ion. ( R-COOH + H20 = H30 + R-COO- ) So formic acid with the addition of water is HCOOH + H20 = H30 + HCOO-
Why is the hydrogen atom of the -OH group on a carboxylic acid the most acidic hydrogen in this type of molecule?
In a carboxylic acid, all hydrogen atoms are attached to carbon atoms except from the one attached to the oxygen atom, on the end of the molecule. This hydrogen ionises on contact with water, producing an H+ ion and an O- ion, which remains attached to the rest of the molecule.Only this hydrogen atom will ever ionise, if hydrogen is attached to carbon it will never ionise, no matter what.
What causes HCl to become an acid?
HCI is the chemical formula for Hydrochloric acid. HCI is composed of a hydrogen ion and a chloride ion. The hydrogen ion causes HCI to become an acid.
Which one is more soluble in water phenol or sodium phenoxide ion?
Carboxylic acid.
IS Hydrogen thiocyanate AN ACID?
Well since it contains hydrogen ion, it must be an acid.
A compound that produces hydrogen ion in solutionis a?
If it produces a hydrogen ion in solution it is referred to as an ACID.
Why is the chemical HI an acid?
It's an acid because it has a hydrogen ion in front of the iodine ion.
What are the products for the addition of a base and a carboxylic acid?
The products are acetate salt with metallic or Ammonium ion and water.
Are acid and hydrogen the same?
An acid is a molecule which, in a solution, separates into a positive and negative ion where the positive ion is one or more hydrogen atoms. Although an acid includes hydrogen atoms, a hydrogen atom or molecule alone is not an acid. Having said that, it can be noted that it is the hydrogen component (which is just the proton part) that makes a compound an acid,
What are Monoprotic acid?
An acid is a substance that will release hydrogen ions (H+) to water or to bases. A monoprotic acid is an acid that has only one hydrogen ion to release per molecule.
